GSA stands for gene specific analysis, the goal of which is to identify the statistical model that is the best for a specific gene among all the selected models, and then use that best model to calculate p-value and fold change.
The first step of GSA is to choose which attributes to include in the test (Figure 1). All sample attributes including numeric and categorical attributes are displayed in the dialog, so use the check button to select between them. An experiment with two attributes Cell type (with groups A and B)and Time (time points 0, 5, 10) is used as an example in this section.
|
Click Next to display the levels of each attribute to be selected for sub-group comparisons (contrasts).
To compare A vs. B, select A for Cell type on the top, B for Cell type on the bottom and click Add comparison. The specified comparison is added to the table below (Figure 2).
|
To compare Time point 5 vs. 0, select 5 for Time on the top, 0 for Time on the bottom, and click Add comparison (Figure 3).
|
To compare cell types at a certain time point, e.g. time point 5, select A and 5 on the top, and B and 5 on the bottom. Thereafter click Add comparison (Figure 4).
|
Multiple comparisons can be computed in one GSA run; Figure 5 shows the above three comparisons are added in the computation.
|
In terms of design pool, i.e. choices of model designs to select from, two 2 factors in this example data will lead to seven possibilities in the design pool:
In GSA, if a 2nd order interaction term is present in the design, then all first order terms must be present, which means, if Cell type * Time interaction is present, the two factors must be included in the model. In the other words, the following designs are not considered:
If a comparison is added, some models that don't have the comparison factors will also be eliminated. E.g. if a comparison on Cell type A vs. B is added, only designs that have Cell type factor included will be in the computation. These are:
The more comparisons on different terms are added, the fewer models will be included in the computation. If the following comparisons are added in one GSA run:
only the following two models will be computed:
If comparisons on all the three terms are added in one GSA run:
then only one model will be computed:
If GSA is invoked from a quantification output data node directly, you will have the option to use the default normalization methods before performing differential expression detection (Figure 6).
|
If advanced normalization needs to be applied, perform the Normalize counts task on a quantification data node before doing differential expression detection (GSA or ANOVA).
Click on Configure to customize Advanced options (Figure 7).
|
Low -expression feature section allows you to specify criteria to exclude features that do not meet requirements for the calculation. If there is filter feature task performed in the upstream analysis, the default of this filter is set to "None", otherwise, the default is Lowest average coverage is set to 1.
Multiple test correction can be performed on the p-values of each comparison, with FDR step-up being the default (1). Other options like Storey q-value (2), and Bonferroni are provided, select one method at a time; None means no multiple test correct will be performed.
FDR step-up:
Suppose there are n p-values (n is the number of features). The p-values are sorted by ascending order and m represents the rank of a p-value. The calculation compares p-value*(n/m) with the specified alpha level, and the cut-off p-value is the one that generates the last product that is less than the alpha level. The goal of step up method is to find:
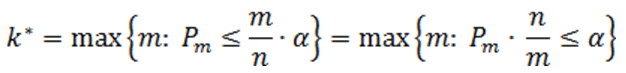
Define the step-up value as:
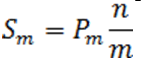
Then an equivalent definition for K* is :
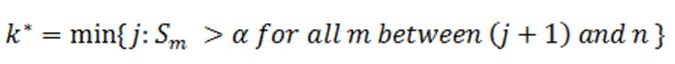
So when
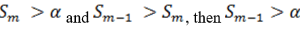
the step up value is
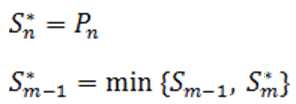
In order to find K* , start with Sn* and then go up the list until you find the first step up value that is less or equal to alpha.
Storey q-value:
q-value is the minimum "positive false discovery rate" (pFDR) that can occur when rejecting a statistic.
For an observed statistic T=t and nested set of rejection area {C},
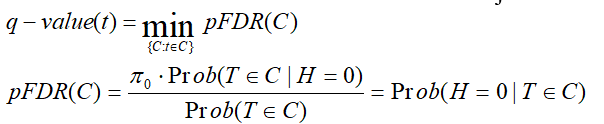
Bonferroni:
Suppose there are n p-values (n is the number of features), the expected number of Type I errors would be given by ![]() , thus the significance level of each individual test should be adjusted to
, thus the significance level of each individual test should be adjusted to ![]() . Alternatively the p-values should be adjusted as pB=p*n, pB is Bonferroni corrected p-value. If pB is greater than 1, it is set to 1
. Alternatively the p-values should be adjusted as pB=p*n, pB is Bonferroni corrected p-value. If pB is greater than 1, it is set to 1
In the results, the best model's Akaike weight is also generated. The model's weight is interpreted as the probability that the model would be picked as the best if the study were reproduced. The range of Akaike weight is between 0 to 1, where 1 means the best model is very superior to the other candidates from the model pool; if the best model's Akaike weight is close to 0.5 on the other hand, it means the best model is likely to be replaced by other candidates if the study were reproduced. One still uses the best shot model, however, the accuracy of the best shot is fairly low.
The default value for Enable multimodel approach is Yes. It means that the estimation will utilize all models in the pool by assigning weights to them based on AIC or AICc. If No is selected instead, the estimation is based on only one best model which has the smallest AIC or AICc. The output p-value will be different depending on the selected option for multimodel, but the fold change is the same. Multimodel approach is recommended when the best model's Akaike weight is not close to 1, meaning that the best model is not compelling.
There are situations when a model estimation procedure does not outright fail, but still encounters some difficulties. In this case, it can even generate p-value and fold change for the comparisons, but those values are not reliable, and can be misleading. It is recommended to use only reliable estimation results, so the default option for Use only reliable estimation results is set Yes.
Partek® Flow® provides five response distribution types for each design model in the pool, namely:
We recommend to use lognormal with shrinkage distribution (the default), and an experienced user may want to click on Custom to configure the model type and p-value type (Figure 8).
|
If multiple distribution types are selected, then the number of total models that is evaluated for each feature is the product of the number of design models and the number of distribution types. In the above example, suppose we have only compared A vs B in Cell type as in Figure 2, then the design model pool will have the following three models:
If we select Lognormal with shrinkage and Negative binomial, i.e. two distribution types, the best model fit for each feature will be selected from 3 * 2 = 6 models using AIC or AICc.
The design pool can also be restricted by Min error degrees of freedom. When "Model types configuration" is set to Default , this is automated as follows: it is desirable to keep the error degrees of freedom at or above six. Therefore, we automatically set to the largest k, 0 <= k <=6 for which admissible models exist. Admissible model is one that can be estimated given the specified contrasts. In the above example, when we compare A vs B in Cell type, there are three possible design models. The error degree of freedom of model Cell type is largest and the error degree of freedom of model Cell type, Time, Cell type * Time is the smallest:
k(Cell type) > k(Cell type, Time) > k (Cell type, Time, Cell type*Time)
If the sample size is big, k >=6 in all three models, all the models will be evaluated and the best model will be selected for each feature. However, if the sample size is too small, none of the models will have k >=6, then only the model with maximal k will be used in the calculation. If the maximal k happens to be zero, we are forced to use Poisson response distribution only.
There are two types of p-value, F and Wald., Poisson, negative binomial and normal models can generate p-value using either Wald or F statistics. Lognormal models always employ the F statistics; the more replicates in the study, the less the difference between the two options. When there are no replicates, only Poisson can be used to generate p-value using Wald.
Note: Partek Flow keeps tracking the log status of the data, and no matter whether GSA is performed on logged data or not, the LSMeans, ratio and fold change calculation are always in linear scale. Ratio is the ratio of the two LSMeans from the two groups in the comparison (left is the numerator, right is the denominator); Fold change is converted from ratio: when ratio is greater than 1, fold change is same as ratio; when ratio is less than one, fold change is -1/ratio. In other words - fold change value is always >=1 or <=-1, there is no fold change value between -1 and 1. When the LSmean of numerator group is greater than that of denominator group, fold change is greater than 1; when LSmean of numerator group is less than denominator group, fold change is less than 1; when the group groups are the same, fold change is 1. Logratio is ratio is log2 transformed, which is equivalent to logfoldchange is some other software.
If there are multiple design models and multiple distribution types included in the calculation, the fraction of genes using each model and type will be displayed as pie charts in the task result (Figure 9).
|
Feature list with p-value and fold change generated from the best model selected is displayed in a table with other statistical information (Figure 10). By default, the gene list table is sorted by the first p-value column.
|
The following information is included in the table by default:
When you click on the Optional columns link on the top-left corner of the table, extra information will be displayed in the table when select:
Click on View extra details report (![]() ) icon under View section to get more statistical information about the feature. In a case that the task doesn't fail, but certain statistical information is not generated, e.g. p-value and/or fold change of a certain comparison are not generated for some or all feature, click on this icon to get more information by mousing over the read exclamation icon
) icon under View section to get more statistical information about the feature. In a case that the task doesn't fail, but certain statistical information is not generated, e.g. p-value and/or fold change of a certain comparison are not generated for some or all feature, click on this icon to get more information by mousing over the read exclamation icon
By clicking on Optional columns, you can retrieve more statistics result information, e.g. Average coverage which is the geometric mean of normalized reads in linear scale across all the samples; fold change lower/upper limits generated from 95% confidence interval; feature annotation information if there are any more annotation fields in the annotation model you specified for quantification, like genomic location, strand information etc.
On the right of each contrast header, there is volcano plot icon ( ![]() ). Select it to display the volcano plot on the chosen contrast (Figure 11).
). Select it to display the volcano plot on the chosen contrast (Figure 11).
|
Feature list filter panel is on the left of the table (Figure 12). Click on the black triangle ( ![]() ) to collapse and expand the panel.
) to collapse and expand the panel.
Select the check box of the field and specify the cutoff by typing directly or using the slider. Press Enter to apply. After the filter has been applied, the total number of included features will be updated on the top of the panel (Result).
Note that for the LSMean, there are two columns corresponding to the two factors in the contrast. The cutoffs can be specified for the left and right columns separately. For example, in Figure 6, the LSMean (left) corresponds to A while the LSMean(right) is for the B.
|
The filtered result can be saved into a filtered data node by selecting the Generate list button at the lower-left corner of the table ( ). Selecting the Download button at the lower-right corner of the table downloads the table as a text file to the local computer.
If lognormal with shrinkage method was selected for GSA, a shrinkage plot is generated in the report (Figure 13). X-axis shows the log2 value of average coverage. The plot helps to determine the threshold of low expression features. If there is an increase before a monotone decrease trend on the left side of the plot, you need to set a higher threshold on the low expression filter. Detailed information on how to set the threshold can be found in the GSA white paper.
|