Page History
| Table of Contents | ||||||
|---|---|---|---|---|---|---|
|
What is trajectory analysis?
Cells undergo changes to transition from one state to another as part of development, disease, and throughout life. These changes can be described by trajectory analysis. Progress though the biological process from the start to an end point is quantified by pseduotime. Because biological processes are rarely as simple as a direct line from one starting point and one end point, trajectory analysis builds branching trajectories where different paths can be chosen at different points along the trajectory.
In Partek Flow, we use tools from Monocle 2 (Qui et al. 2017) to 2 [1] to build trajectories, identify states and branch points, and calculate pseudotime values. The output of Trajectory analysis includes an interactive scatter plot visualization for viewing the trajectory and setting the root state (starting point of the trajectory) and adds a categorical cell level attribute, State. From the Trajectory analysis task report, you can run a second task, Calculate pseudotime, which adds a numeric cell-level attribute, Pseudotime, which is calculated using the chosen root state. Using the state and pseudotime attributes, you can perform downstream analysis to identify genes that change over pseudotime and characterize branch points.
Prerequisites for trajectory analysis
For Trajectory analysis to work as expected, there are a few things you do prior to running it.Note that trajectory analysis will only work on data with <600,000,000 observations (number of cells × number of features). If your data set exceeds this limit, the Trajectory analysis task will not appear in the toolbox. Prior to performing trajectory analysis, you should:
1) Normalize the data
Trajectory analysis requires normalized counts as the input data. We recommend our default "CPM, Add 1, Log 2" normalization for most scRNA-Seq data. For alternative normalization methods, see our Normalization documentation.
2) Filter to cells that belong in the same trajectory
Trajectory analysis will built build a single branching trajectory for all input cells. Consequently, only cells that share the biological process being studied should be included. For example, a trajectory describing progression through T cell activation should not include monocytes that do not undergo T cell activation. To learn more about filtering, please see our Filter groups (samples or cells) documentation.
3) Filter to genes that characterize the trajectory
The trajectory should be built using a set of genes that increase or decrease as a function of progression through the biological processes being modeled. One example is using differentially expressed genes between cells collected at the beginning of the process to cells collected at the end of the process. If you have no prior knowledge about the process being studied, you can try identifying genes that are differentially expressed between clusters of cells or genes that are highly variable within the data set. Generally, you should try to filter to 1,000 to 3,000 informative genes prior to running Trajectory performing trajectory analysis. The list manager functionality in Partek Flow is useful for creating a list of genes to use in the filter. To learn more, please see our documentation on Lists.
Parameters
Two parameters are exposed for trajectory analysis. First, the number of dimensions. Dimensionality of the reduced space
While the trajectory is always visualized in a 2D scatter plot, the underlying structure of the trajectory may be more complex and better represented by more than 2 two dimensions. Second, you
Scaling
You can choose to scale the genes prior to building the trajectory. Scaling removes any differences in variability between genes, while not scaling allows more variable genes to have a greater weight in building the trajectory.
Task report
The Trajectory analysis Click on the task report is , a 2D scatter plot. scatterplot will be opened in Data viewer (Figure 1).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
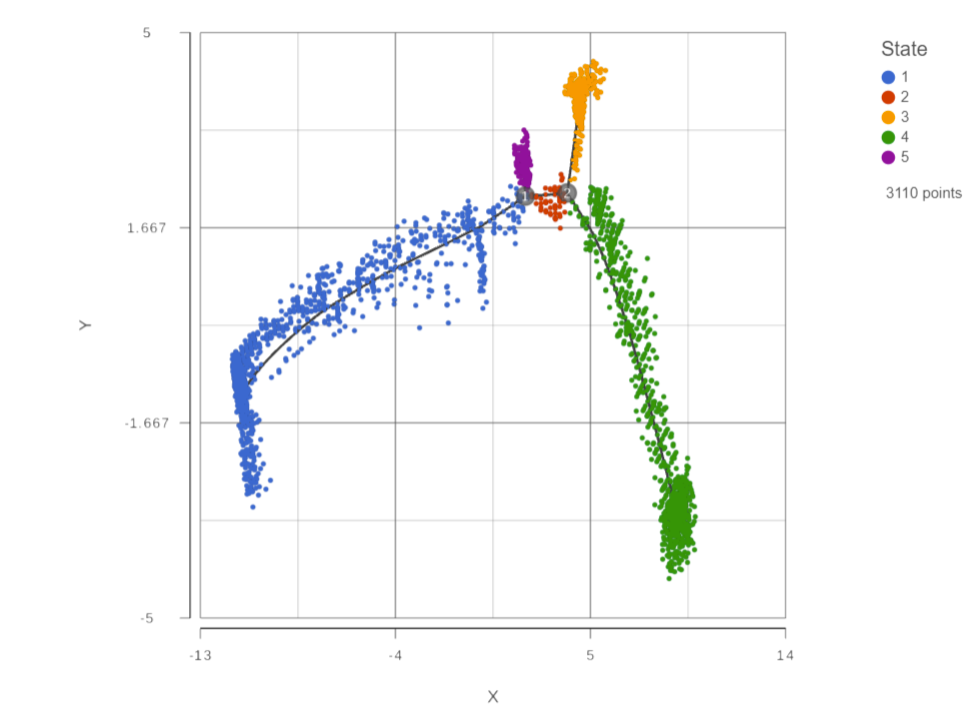
Figure 1: Trajectory report is a 2D scatterplot
The trajectory is shown with a black line showing the trajectory, numbers at each branch point, and the cells . Branch points are indicated by numbers in black circles. By default, cells are colored by state. Like any scatter plot, you can color You can use the control panel on the left to color, size, and shape by genes and attributes to help identify which state is the root or origin of the trajectory. To select a root state, click
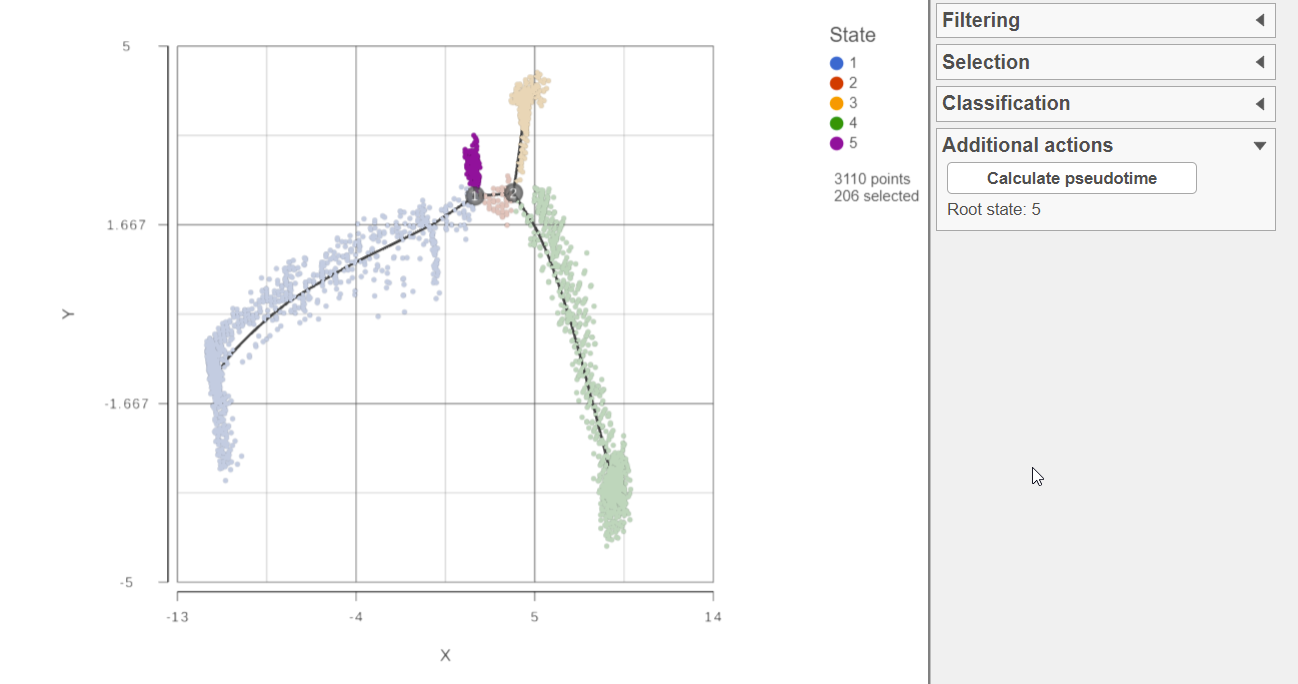
Calculating pseudotime
To calculate pseudotime, you must choose a root state. The tip of the root state branch will have a value of 0 for pseudotime. Click any cell belonging to that state . Clicking Calculate pseudotime will run the task to calculate pseudotime values for each cell using the selected state as the root.
| Additional assistance |
|---|
|
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
. |
Figure 2: Select a root state to calculate pseudotime
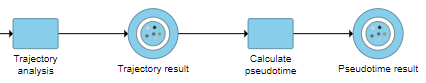
The Calculate pseudotime task will be performed, it generates a new Pseudotime result data node, which contains Pseudotime annotation for each cell (Figure 3).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
Figure 3: Perform Pseudotime calculation task to generate pseudotime result
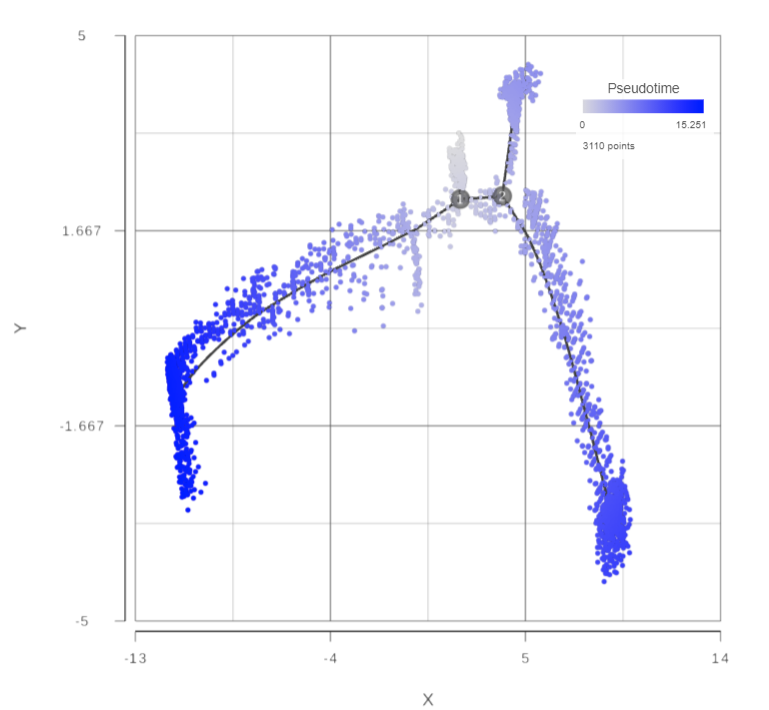
Open the Pseudotime result report, a 2D scatterplot will be displayed in data viewer, colored by Pseudotime by default (Figure 4)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
This will run a task on the analysis pipeline, Calculate pseudotime, and output a new Pseudotime result data node (Figure 6).
References
[1] Xiaojie Qiu, Qi Mao, Ying Tang, Li Wang, Raghav Chawla, Hannah Pliner, and Cole Trapnell. Reversed graph embedding resolves complex single-cell developmental trajectories. Nature methods, 2017.
| Additional assistance |
|---|
| Rate Macro | ||
|---|---|---|
|
...