Page History
...
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
- Click Settings
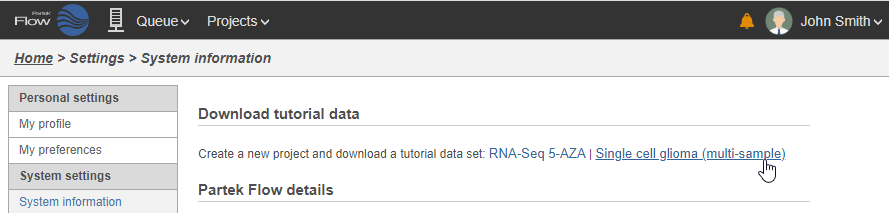
On the System information page, the Download tutorial data section includes pre-loaded data sets used by Partek Flow tutorials (Figure 2).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
- Click Single cell glioma (multi-sample)
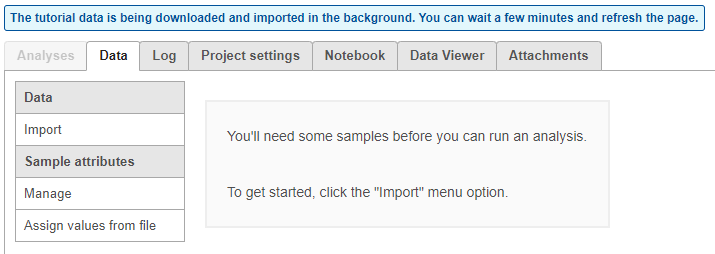
The tutorial data set will be downloaded onto your Partek Flow server and a new project, Glioma (multi-sample), will be created. You will be directed to the Data tab of the new project. Because this is a tutorial project, there is no need to click on Import data, as the import is handled automatically (Figure 3).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
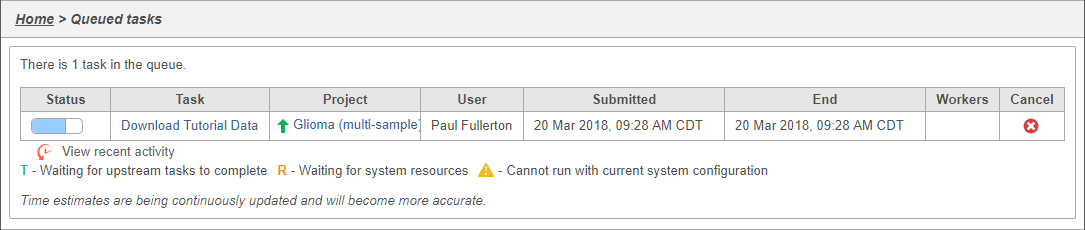
You can wait a few minutes for the download to complete, or check the download progress by selecting Queue then View queued tasks... to view the Queue (Figure 4).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
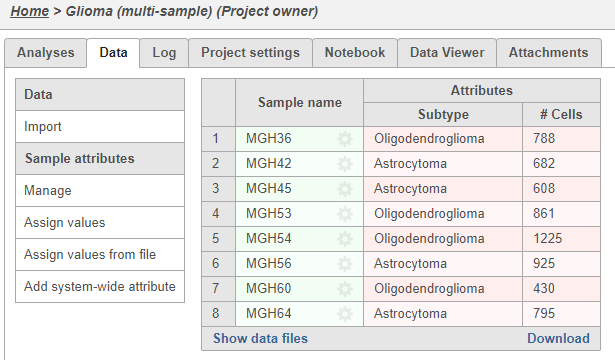
Once the download completes, the sample table will appear in the Data tab , with one row per sample (Figure 5).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
...
Annotating samples with attributes
The Data tab displays the samples in the project with the number of cells in each sample (Figure 5). One of the goals of this analysis will be to compare gene expression in a cell type between the two Glioma subtypes. For this, we need to add an annotation indicating the subtype of each sample.
- Click Manage attributes
- Click Add new attribute (Figure 6)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
- Type Subtype in the Name text field
- Click Add (Figure 7)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
- Type Astrocytoma in the New category text field
- Click Add
- Type Oligodendroglioma in the New category text field
- Click Close
- Click Back to sample management table
There is new column, Subtype, in the Data tab, but every sample has a value of N/A. Next, we will assign each sample to a subtype.
- Click Edit attributes
Use the drop-down menus to assign each sample to its corresponding subgroup (Figure 8)
Sample Name Subtype MGH36 Oligodendroglioma MGH42 Astrocytoma MGH45 Astrocytoma MGH53 Oligodendroglioma MGH54 Oligodendroglioma MGH56 Astrocytoma MGH60 Oligodendroglioma MGH64 Astrocytoma
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
- Once each sample has been assigned to a subgroup, click Apply changes to proceed
Filtering cells in single cell RNA-Seq data
...
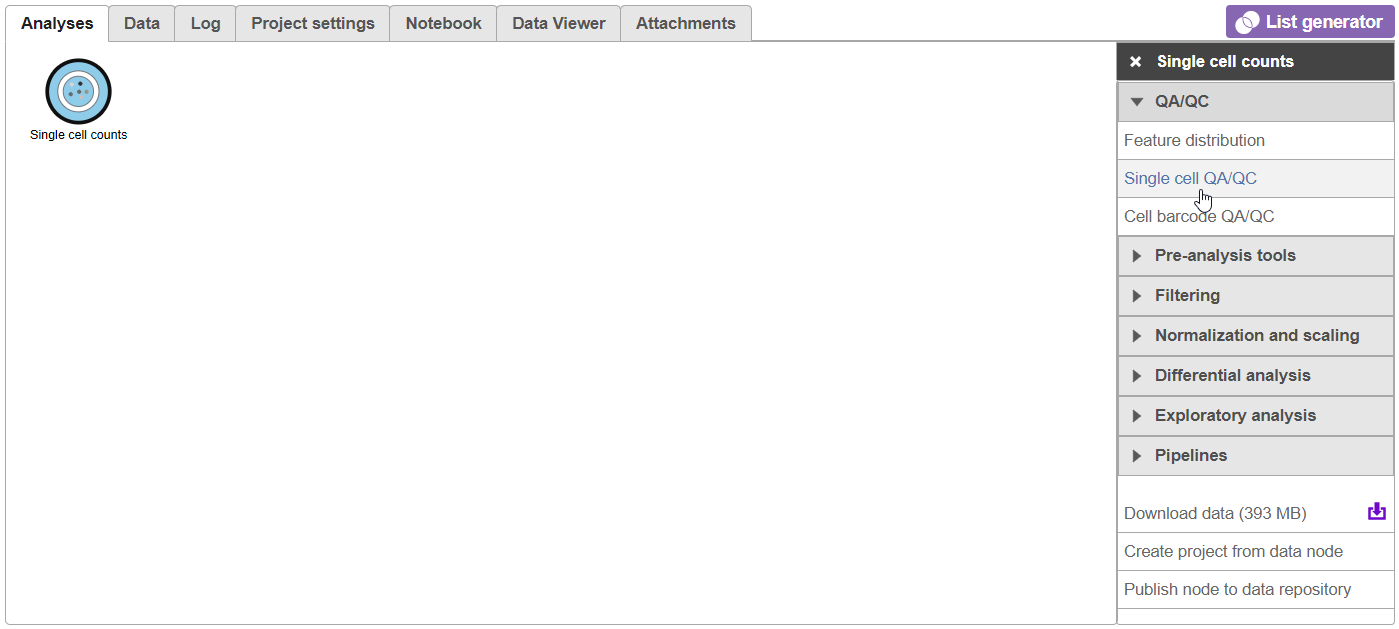
For now, the Analyses tab has only a single node, Single cell countsdata. As you perform the analysis, additional nodes representing tasks and new data will be created, forming a visual representation of your analysis pipeline.
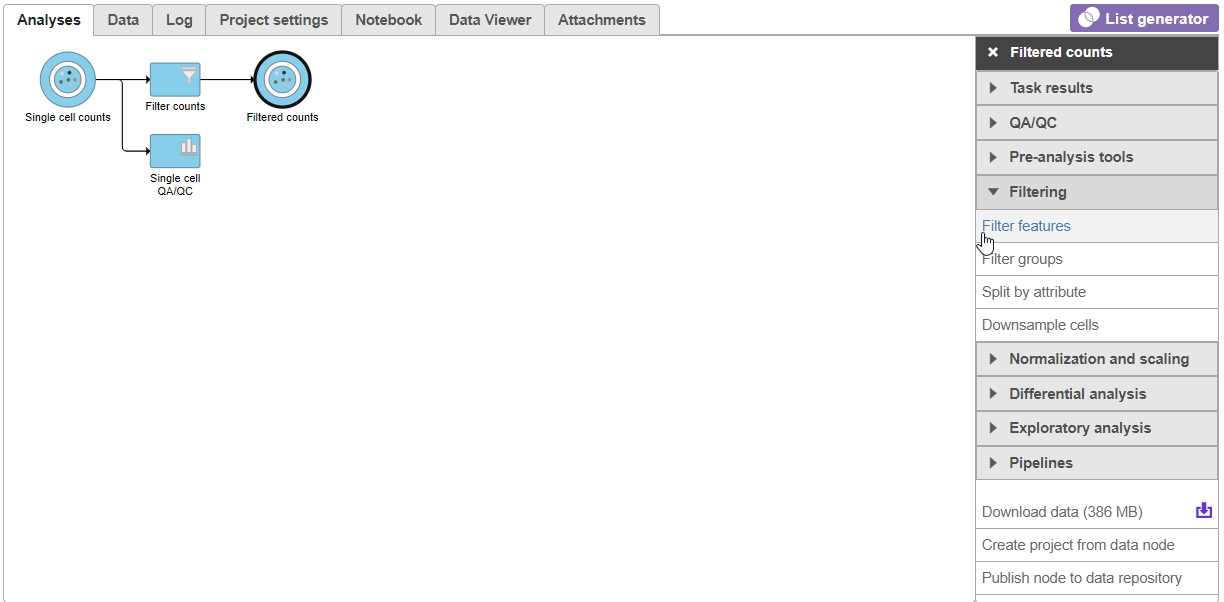
- Click on the Single cell counts data node
A context-sensitive menu will appear on the right-hand side of the pipeline (Figure 9). This menu includes tasks that can be performed on the selected counts data node.
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
An important step in analyzing single cell RNA-Seq data is to filter out low - quality cells. A few examples of low-quality cells are doublets, cells damaged during cell isolation, or cells with too few counts reads to be analyzed.
- Expand the Click on QA/QC section of the task menu
- Click on Single cell QA/QC (Figure 610)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
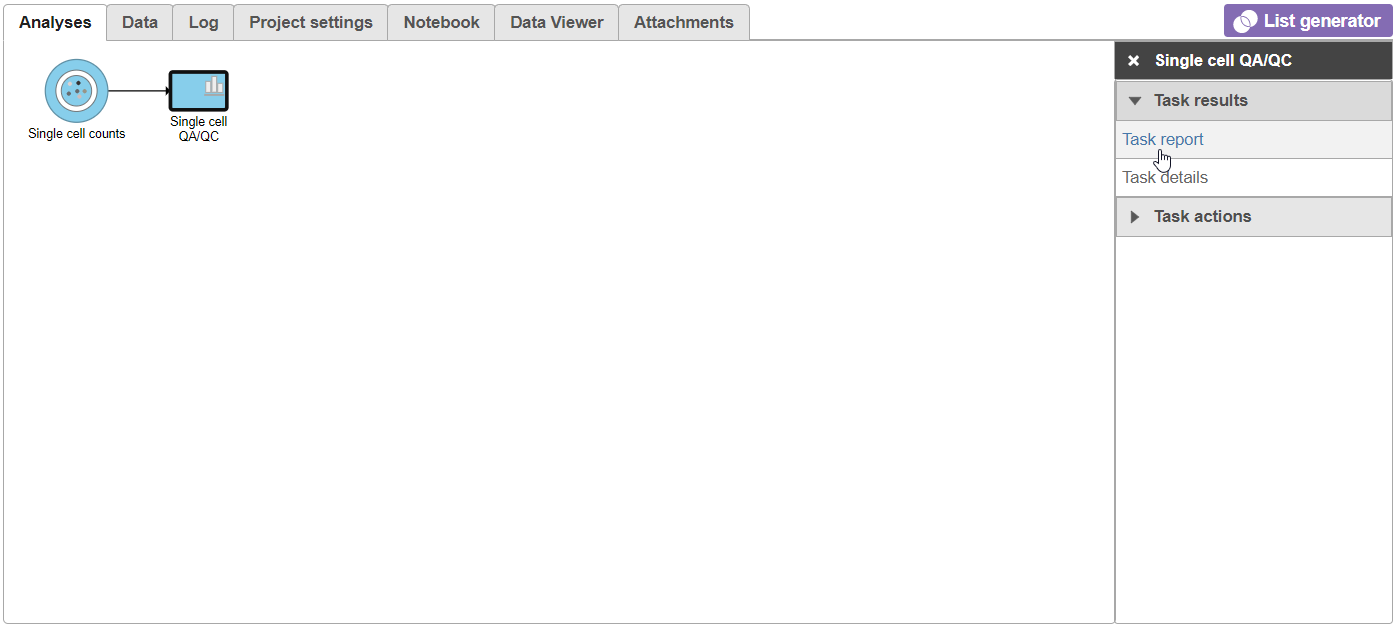
A task node, Single cell QA/QC, is produced. Initially, the node will be semi-transparent to indicate that it has been queued, but not completed. A progress bar will appear on the Single cell QA/QC task node to indicate that the task is running.
- Click the Single cell QA/QC node once it finishes running
- Click Task report on the task menu (Figure 7Figure 11)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
The Single cell QA/QC report opens in a new data viewer session. There are includes interactive violin plots showing the most commonly used quality metrics for each cell from all samples combined (Figure 8). For value of every cell in the project on several quality measures (Figure 12).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
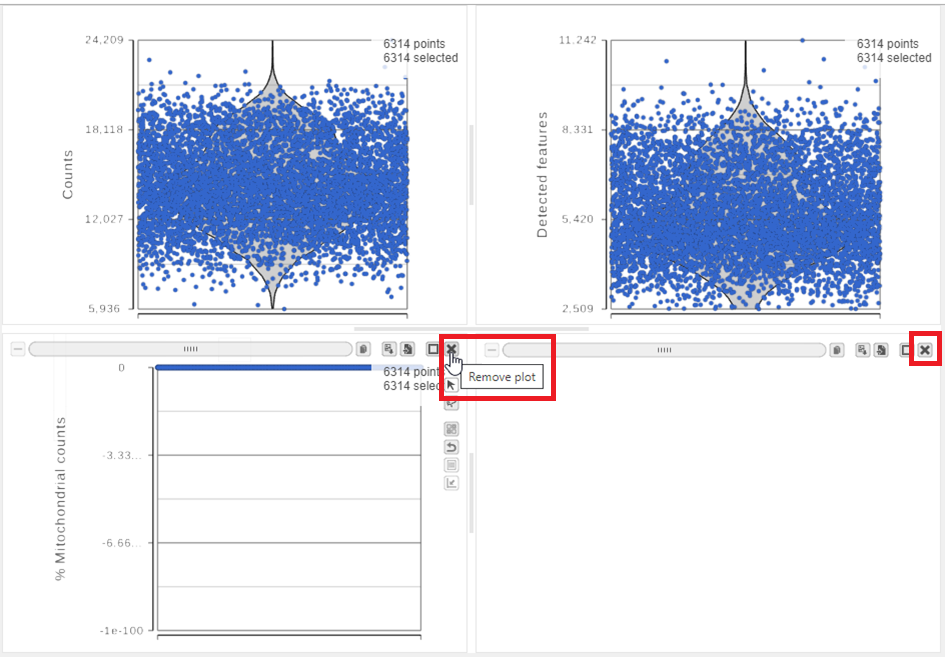
For this data set, there are two relevant plots: the total count number of reads per cell and the number of detected genes per cell. Each point on the plots is a cell and the violins illustrate the distribution of values for the y-axis metric. Typically, there is a third plot showing the percentage of mitochondrial counts reads per cell, but mitochondrial transcripts were not included in the data set by the study authors, so this plot is not informative for this data set.
- Remove the % mitochondrial counts and the extra text box in the bottom right by clicking Remove plot in the top right corner of each plot (Figure 8).
...
Each
...
point on the
...
The plots are highly customizable and can be used to explore the quality of cells in different samples.
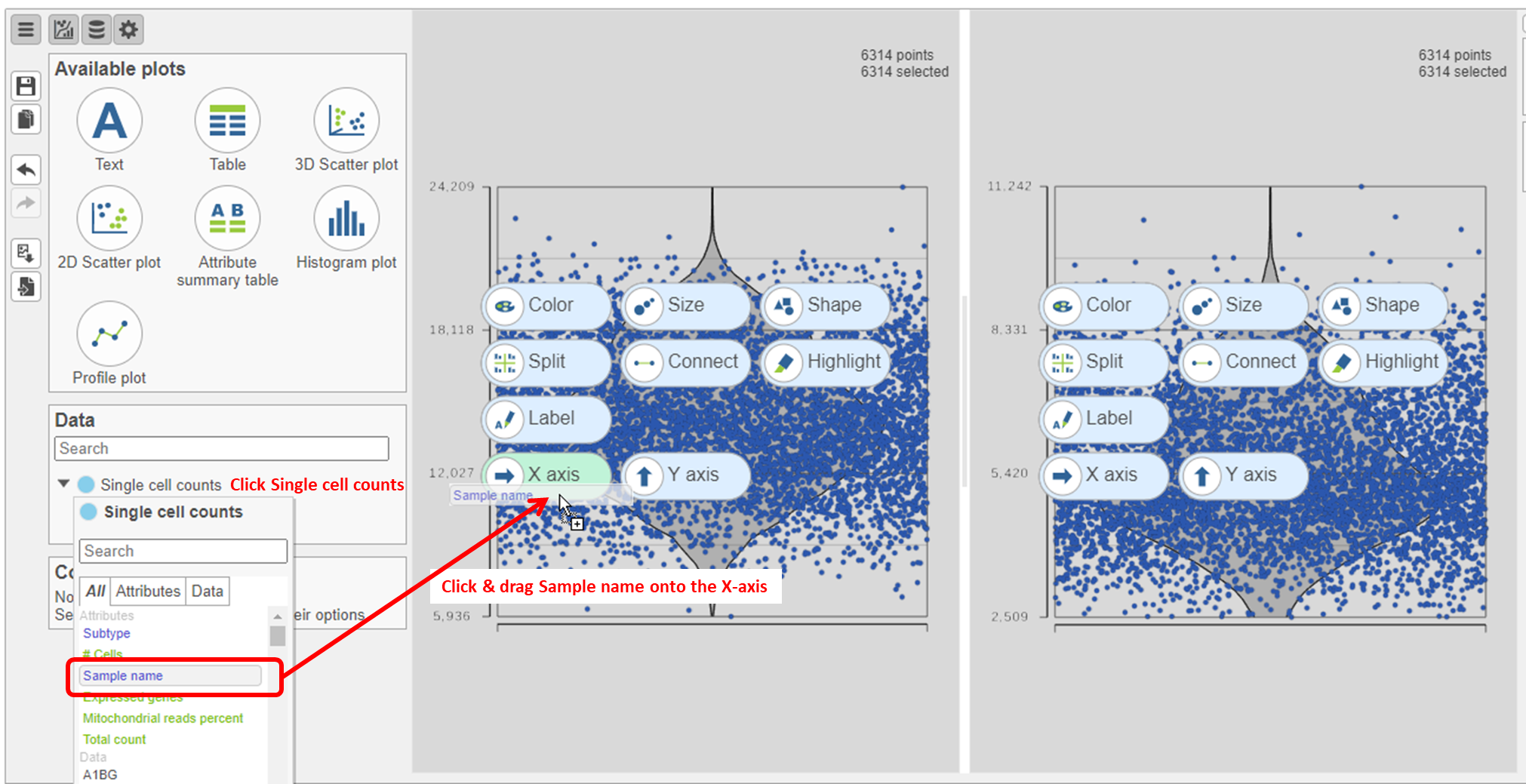
- Click on Single cell counts in the Data card on the left (Figure 9)
- Click and drag the Sample name attribute onto the Counts plot and drop it onto the X-axis
- Repeat this for the Detected genes plot
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
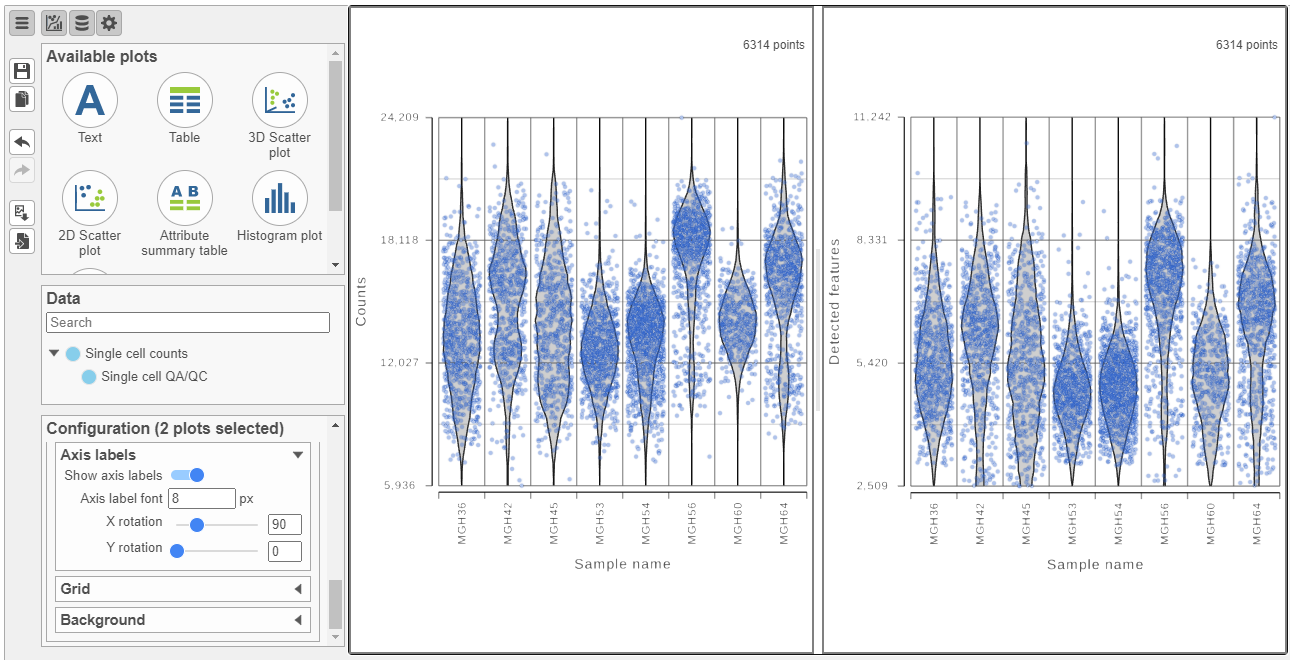
The cells are now separated into different samples along the x-axis (Figure 10)
- Hold Control and left-click to select both plots
- In the Configuration card on the left, scroll down and expand the Color card
- Use the slider to reduce the Opacity
- In the Configuration card on the left, scroll down and expand the Axis label card
- Adjust the X-rotation on the plots to 90
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
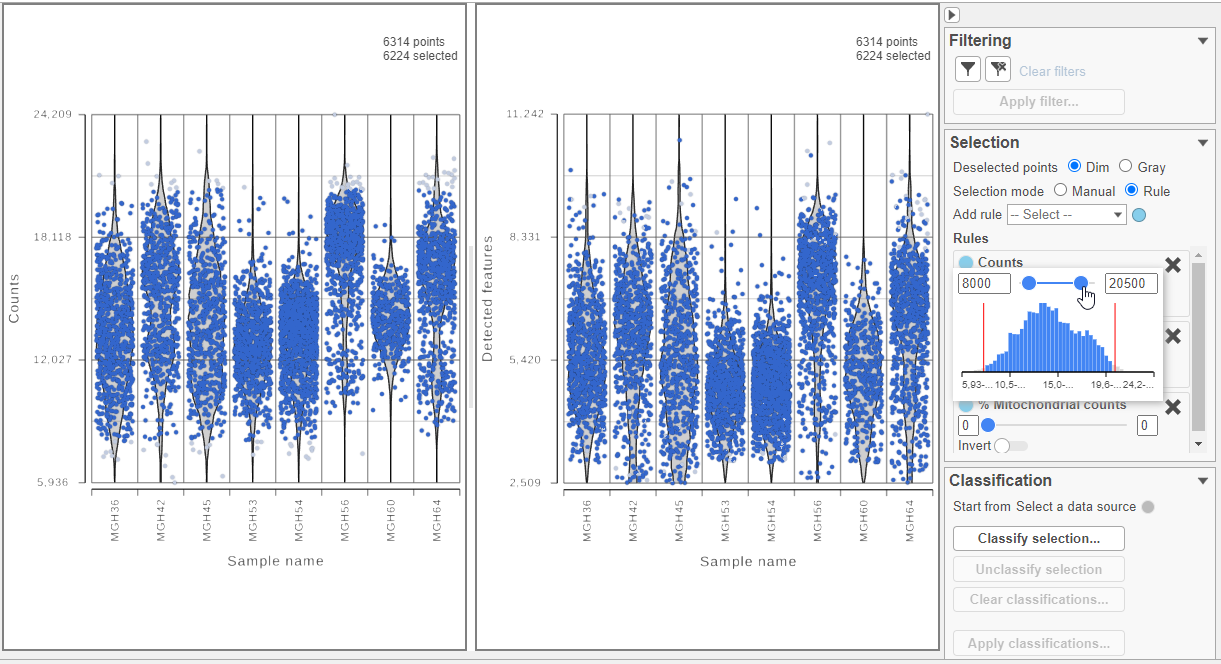
Cells can be selected by setting thresholds in the Selection card on the right. Here, we will select cells based on the total count
- Set the Counts thresholds to 8000 and 20500
Selected cells will be in blue and deselected cells will be dimmed (Figure 11).
plots is a cell and the violins illustrate the distribution of values for the y-axis metric. Cells can be filtered either by clicking and dragging to select a region on one of the plots or by setting thresholds using the filters below the plots. Here, we will apply a filter for the number of read counts.
- Set the Read counts filter to Keep cells between 8000 and 20500 reads
The plot will be shaded to reflect the filter. Cells that are excluded will be shown as black dots on both plots (Figure 13).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
Because this data set was already filtered by the study authors to include only high-quality cells, this count read counts filter is sufficient.
- Click in the Filtering card on the right
- Click Apply filter
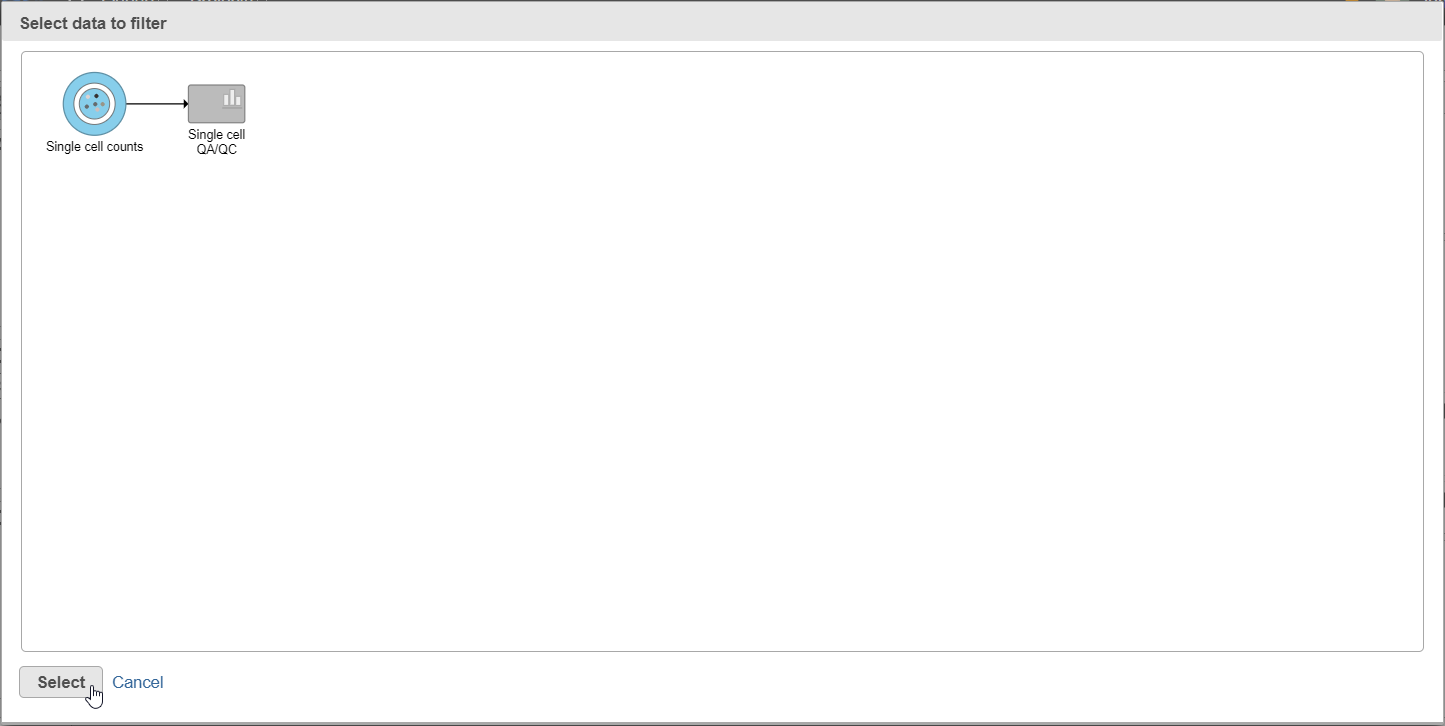
- Click the Single cell counts data node in the pipeline preview (Figure 12)
- Click Select
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
- Click Apply filter
A new task, Filter countscells, is added to the Analyses tab. This task produces a new Filter counts data node (Figure 13).
- Click on the Glioma (multi-sample) project name at the top to go back to the Analyses tab
- Your browser may warn you that any unsaved changes to the data viewer session will be lost. Ignore this message and proceed to the Analyses tab
Single cell data node (Figure 14).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
Most tasks can be queued up on data nodes that have not yet been generated, so you can wait for filtering step to complete, or proceed to the next section.
...
A common task in bulk and single-cell RNA-Seq analysis is to filter the data to include only informative genes. Because there is no gold standard for what makes a gene informative or not , and ideal gene filtering criteria depends depend on your experimental design and research question. Thus, Partek Flow has a wide variety of flexible filtering options.
- Click the Filter counts Single cell data node produced by the Filter counts cells task
- Click Filtering in the task menu
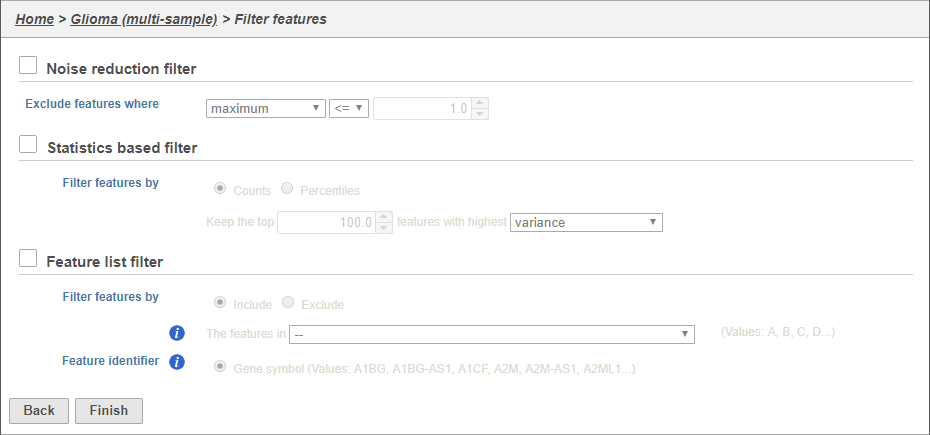
- Click Filter features (Figure 14Figure 15)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
There are three categories of filter available - noise reduction, statistics based, and feature list (Figure 15Figure 16).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
We will use a noise reduction filter to exclude genes that are not expressed by any cell in the data set, but were included in the matrix file.
- Click the Noise reduction filter checkbox check box
- Set the Noise reduction filter to Exclude features where value <= 0 in 99% of cells using the drop-down menus and text boxes (Figure 16)
- Click Finish to apply the filter
...
The tutorial data set is taken from a published study and has already been normalized using TPM (Transcripts per million), which normalizes for the length of feature and total reads, and transformed as log2(TPM/10+1). This normalization and transformation scheme can be performed in Partek Flow, along with other commonly used RNA-Seq data normalization methods.
For more information on normalizing data in Partek Flow, please see the Normalize counts section of the user manual.
| Page Turner | ||
|---|---|---|
|
| Additional assistance |
|---|
|
| Rate Macro | ||
|---|---|---|
|