Page History
| Table of Contents | ||
|---|---|---|
|
What is Cell Ranger?
Cell Ranger is a set of analysis pipelines that process Chromium single cell data to align reads, generate feature-barcode matrices and perform clustering and gene expression analysis for 10X Genomics Chromium Technology[1].
Cell Ranger - ATAC in Partek Flow
Cell Ranger - ATAC task in Partek® Flow® includes two different wrappers. To deal with the single cell ATAC-seq dataset, the 'cellranger-atac count' pipeline from Cell Ranger ATAC v2.0[2] has been wrapped in Flow®. It takes FASTQ files from 'cellranger-atac mkfastq' and performs ATAC analysis including reads filtering and alignment, barcode counting, identification of transposase cut sites, peak and cell calling, count matrix generation. Its outputs then become the starting point for downstream analysis for scATAC-seq data in Flow. To process Chromium Single Cell Multiome ATAC + Gene Expression sequencing data, ‘cellranger-arc count’ v2.0[3] has been wrapped to generate a variety of analyses pertaining to gene expression, chromatin accessibility and their linkage.
Running Cell Ranger - ATAC in Flow
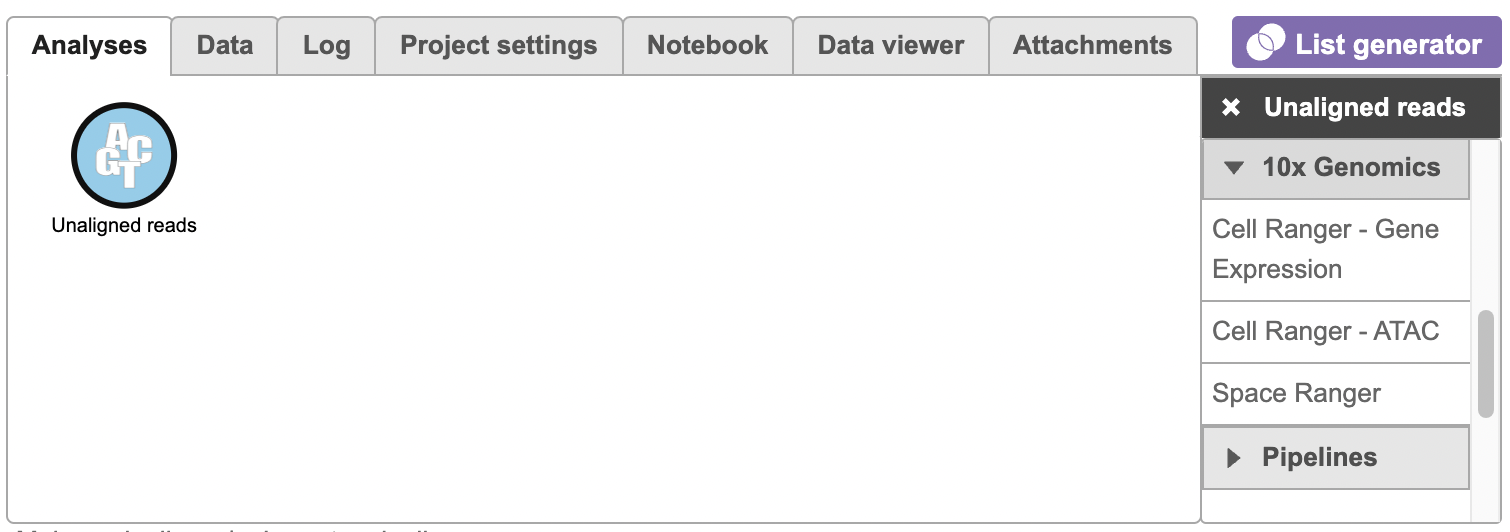
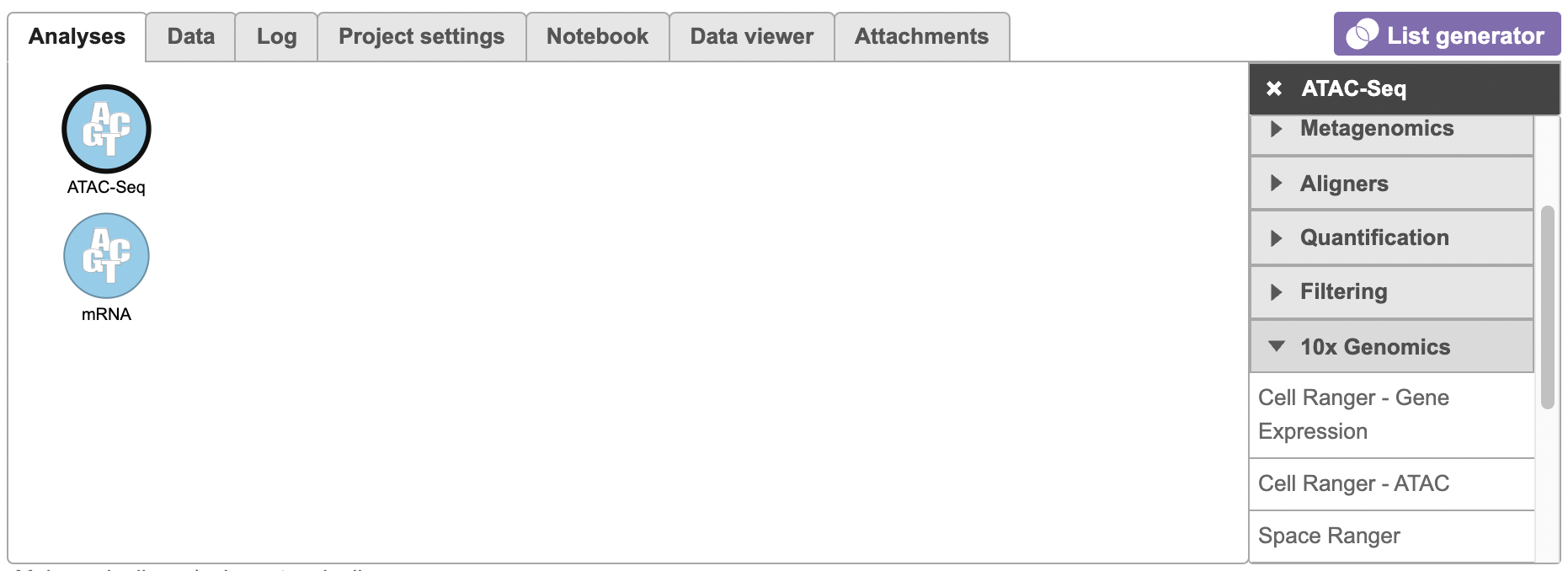
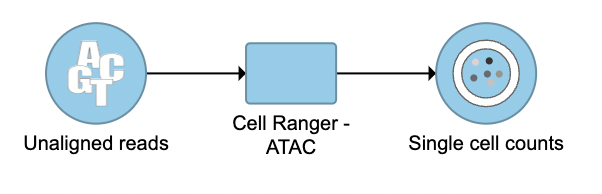
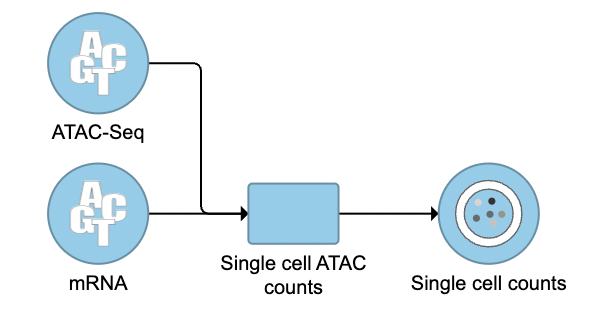
To run the Cell Ranger - ATAC task for scATAC-seq data in Flow, select Unaligned reads datanode, then select Cell Ranger - ATAC in the 10x Genomics section (left panel, Figure 1). For 10x multiome ATAC + Gene Expression data, there will be two data nodes once the FASTQ files have been imported into Flow properly - ATAC-Seq and mRNA (right panel, Figure 1). Users should select ATAC-Seq datanode to trigger the Cell ranger - ATAC task.
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
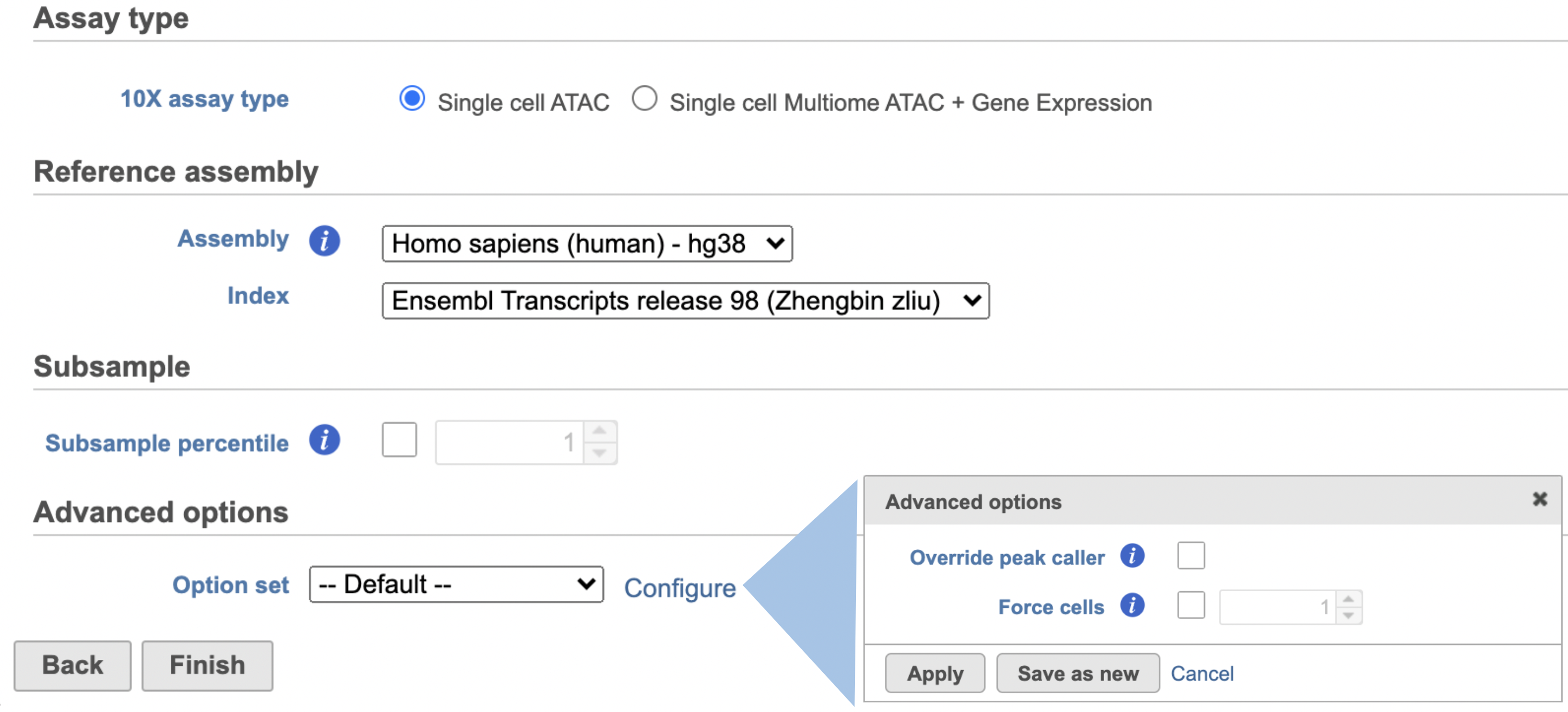
Similar to Cell Ranger - Gene Expression task, the first time user will be asked to create a Reference assembly. In Partek® Flow®, we will use Cell Ranger ARC 2.0.0 to create reference assembly for all 10x Genomics analysis pipelines. Please refer to our Cell Ranger - Gene Expression task manual on how to build or use Reference assembly.
Once the right assembly has been chosen/provided, simply press the Finish button to run the task with default settings. The reference assembly of ‘Homo sapiens (human) - hg38’ has been used as an example here (Figure 2).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
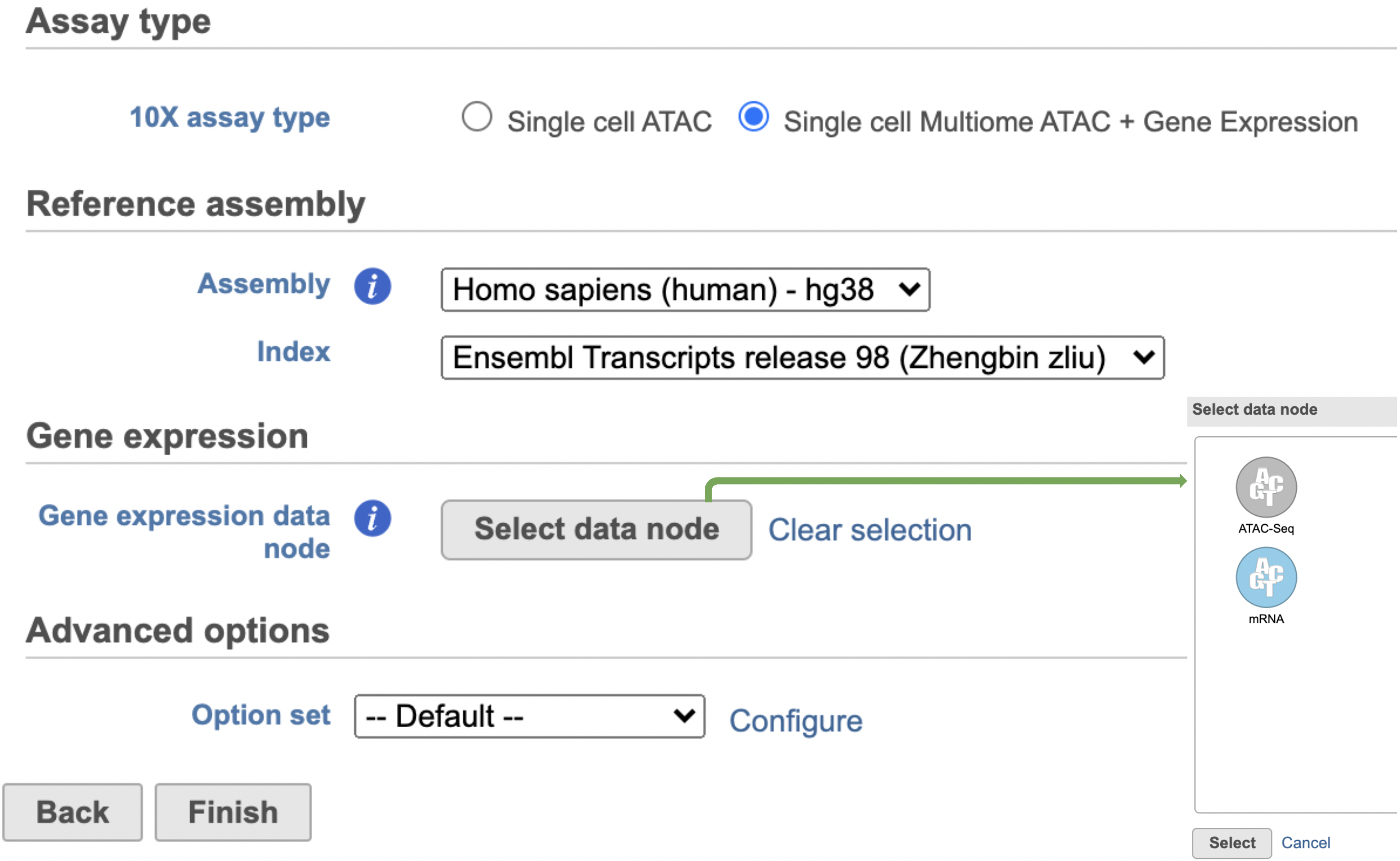
The interface will be different for Single Cell Multiome ATAC + Gene Expression sequencing data because the gene expression data from the very same cell has to be paired with the ATAC-Seq data (Figure 3)
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
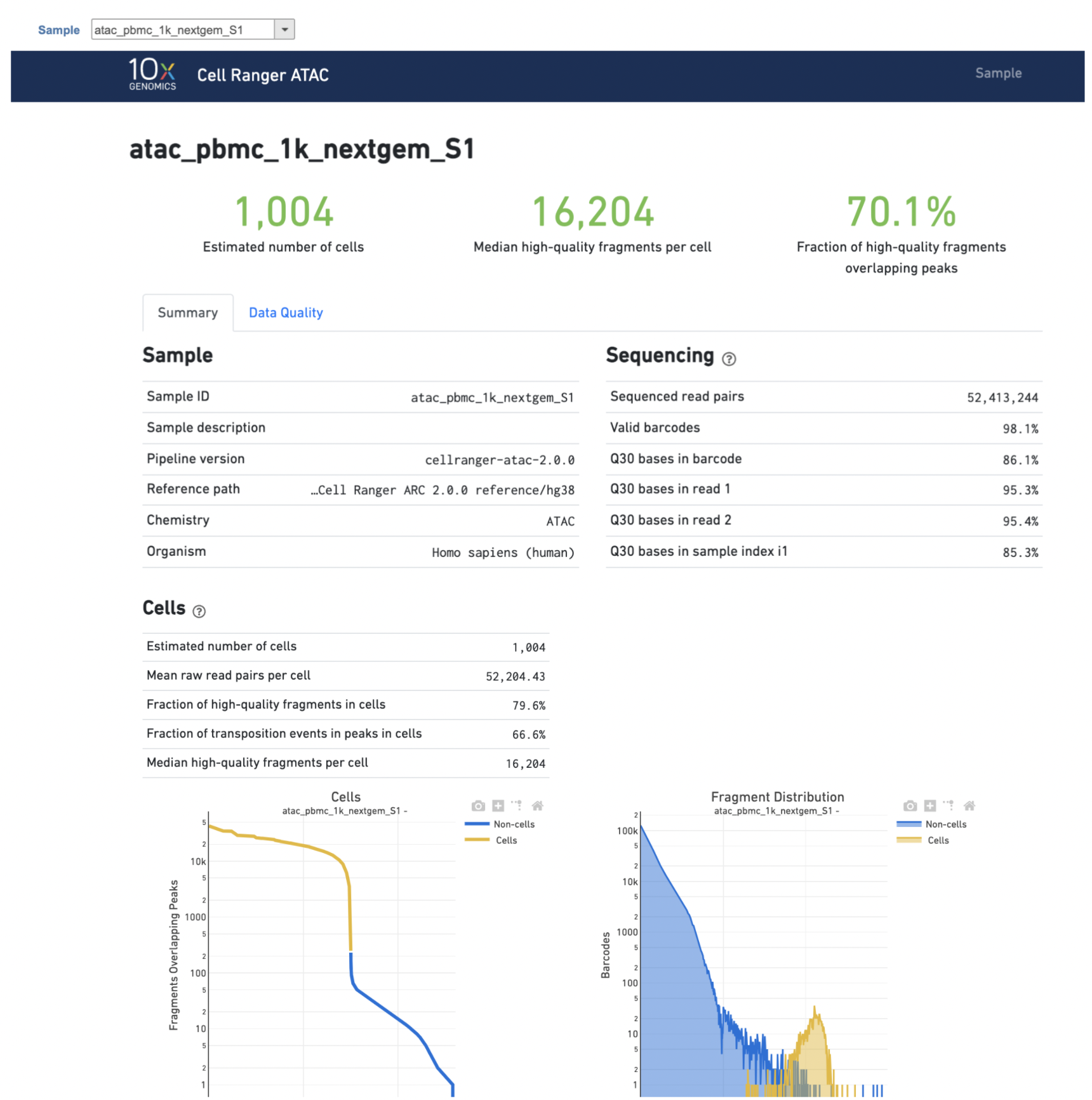
A new data node named Single cell counts will be displayed in Flow if the task has been finished successfully (Figure 4). This data node contains a filtered peak barcode count matrix for ATAC-Seq data, but a unified feature-barcode matrix that contains gene expression counts alongside ATAC-Seq peak counts for each cell barcode for multiomic data. To open the task report when the task is finished, double click the output data node, or select the Task report in the Task results section after single clicking the data node. Users then will find the task report (Figure 5) is the same as the ‘Summary HTML’ from Cell Ranger ATAC output.
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
Cell Ranger - ATAC task report in Flow
Task report is sample based. Users can use the dropdown list on the top left to switch samples. Under the sample name, there are two tabs on each report - Summary report and Data Quality report (Figure 5). Important information on Estimated Number of Cells, Median high-quality fragments per cell, Fraction of high-quality fragments overlapping peaks, as well as information on Sample, Sequencing, Cells and Cell Clustering are summarized in different panels. The Barcode Rank plot and the Fragment Distribution plot have also been included as an important piece in the Cells section in the Summary report (Figure 5). Descriptions of metrics in the following sections can also be found by clicking the to the section header in the Summary HTML file itself.
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
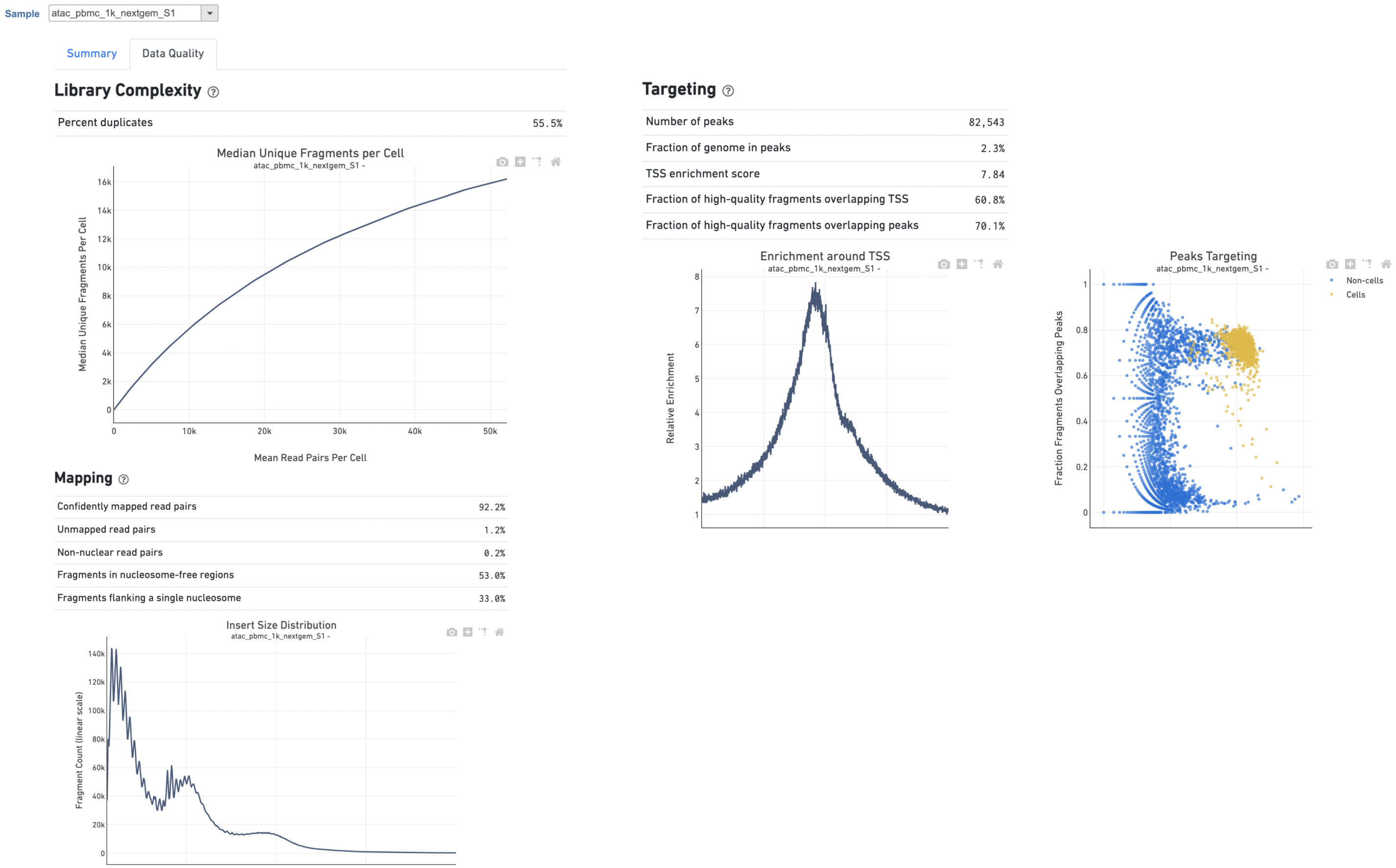
The Library Complexity section in Data Quality report plots the observed per cell complexity, measured as median unique fragments per cell, as a function of mean reads per cell. (Figure 6). While the Mapping section displays the Insert Size Distribution plot, and metrics derived from it. Single Cell ATAC read pairs produce detailed information about nucleosome packing and positioning. The fragment length distribution captures the nucleosome positioning periodicity. The Targeting section shows profiling of the chromatin accessibility behavior of the library at epigenetically relevant regions in the genome. The Enrichment around TSS plot is helpful to assess the signal-to-noise ratio of the library. It is well known that TSSs and the promoter regions around them have a higher degree of chromatin accessibility compared to the other regions of the genome. The Peaks targeting plot presents the variation in the number of on-target fragments, or fragments that overlap peaks, within each barcode group. A higher percentage of the barcode fragments overlap peaks is expected for cell-associated barcodes.
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
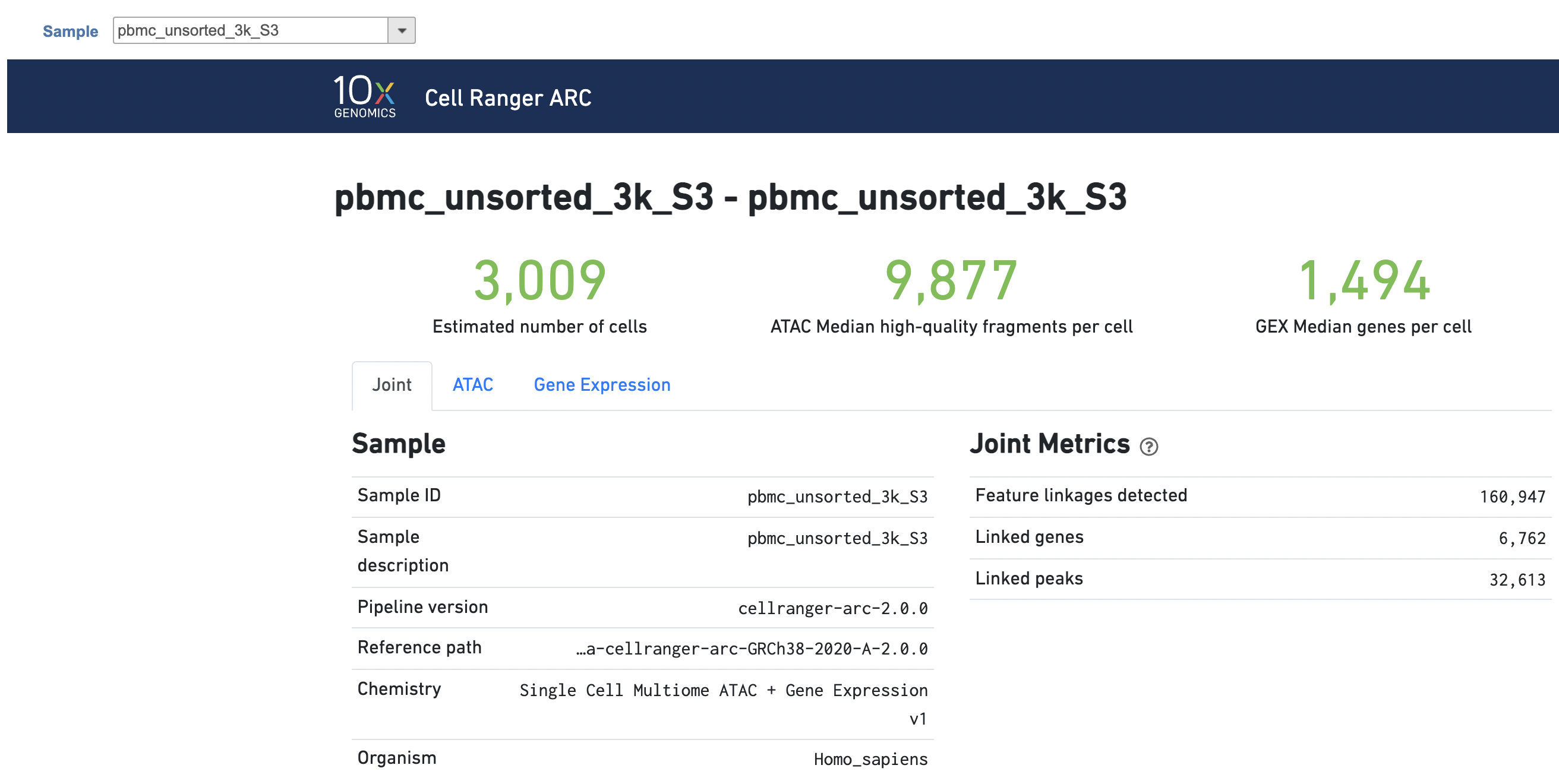
The task report for multiomic data analysis is much more complicated. It contains summary metrics at different levels - ATAC, gene expression, both/joint. Joint view is the default view visible upon first rendering the summary and can be accessed by clicking "Joint" at the top left corner. Metrics that are specific to the given Chromatin Accessibility library will appear in the ATAC tab. Lastly, metrics that are specific to the given gene expression library will appear in the Gene Expression tab (Figure 7). To understand the details, please refer to 10x Genomics webpage[4].
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
Other adjustable parameters in this task (Figure 2) include:
Subsample percentile: Downsample to preserve this fraction of reads.
Users can also click Configure to change the default settings in Advanced options (Figure 2).
Override peak caller: To override the peak caller, users specify peaks to use in downstream analyses from supplied 3-column BED file. The supplied peaks file must be sorted by position and not contain overlapping peaks; comment lines beginning with `#` are allowed.
Force cells: Define the top N barcodes with the most fragments overlapping peaks as cells and override the cell calling algorithm. N must be a positive integer <= 20,000. Use this option if the number of cells estimated by Cell Ranger -ATAC is not consistent with the barcode rank plot.
Memory limit (GB): Restricts Cell Ranger - ATAC to use specified amount of memory (in GB) to execute pipeline stages.
Import Cell Ranger output into Flow
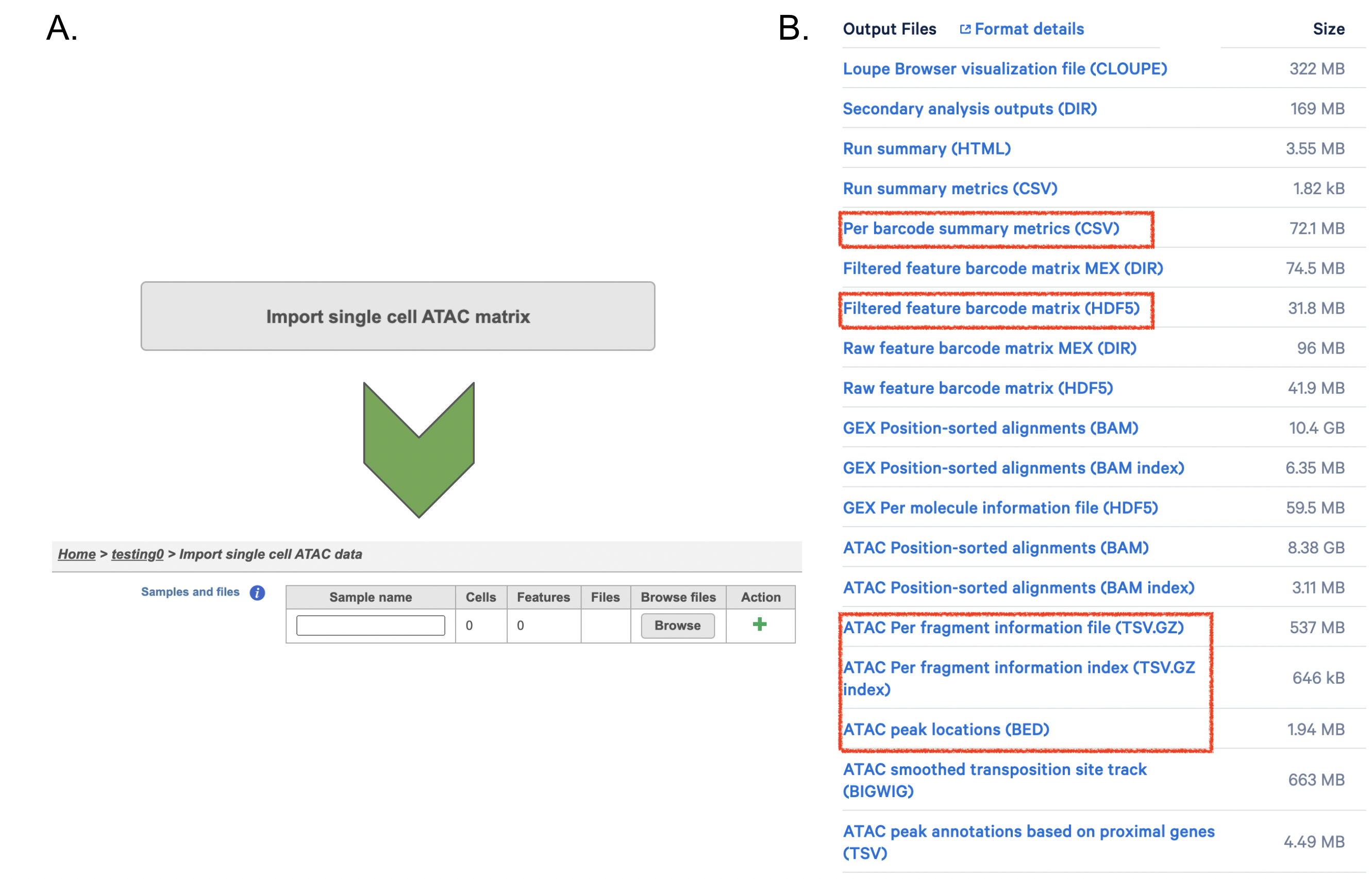
If users have converted FASTQ outside of Partek, Flow has the importer available for count matrix (Figure 8A). Files that Flow will need to complete the import includes the followings:
filtered_feature_bc_matrix.h5
per_barcode_metrics.csv
peaks.bed
fragments.tsv.gz.tbi
fragments.tsv.gz.
Those five files usually could be found in the outs/ subdirectory within the pipeline output directory (Figure 8B). scATAC-seq is complicated, unlike RNA-seq - if peak calling was performed on each sample/dataset independently, the peaks are unlikely to be exactly the same. We therefore need to create a common set of peaks across all the samples/datasets to be merged. The datasets combination in Flow is performed during data import - therefore it needs all samples/datasets to be imported at one time, not separately. Please click the green + button to add more samples (Figure8A).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
Import FASTQs for Cell Ranger -ATAC in Flow
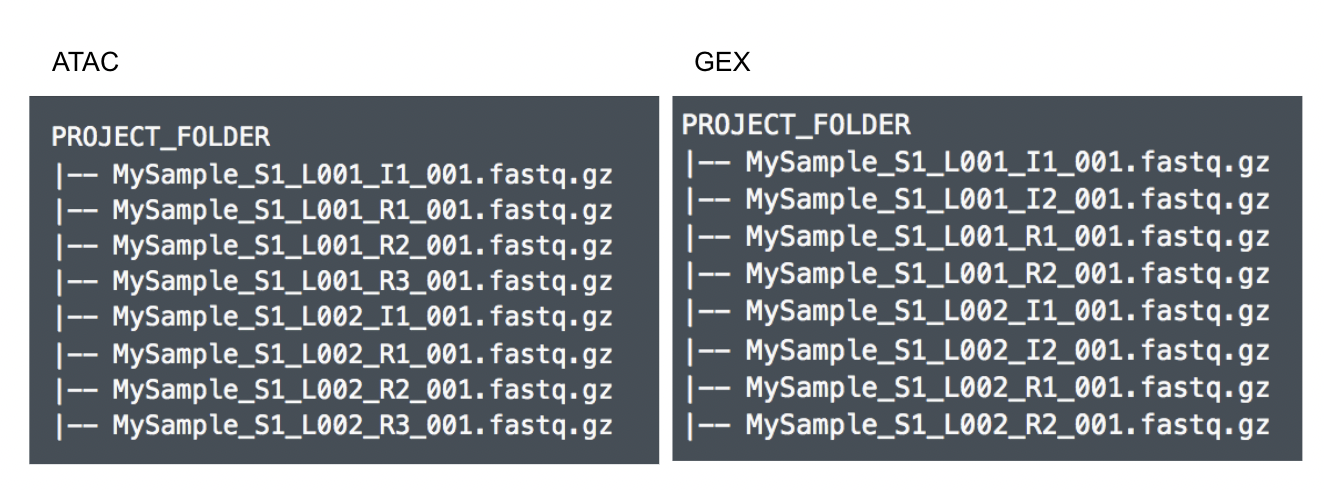
Although the index files (I1 or I2) are optional, we encourage users to include all the FASTQs in the table (Figure 9) while importing data for Cell Ranger - ATAC.
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
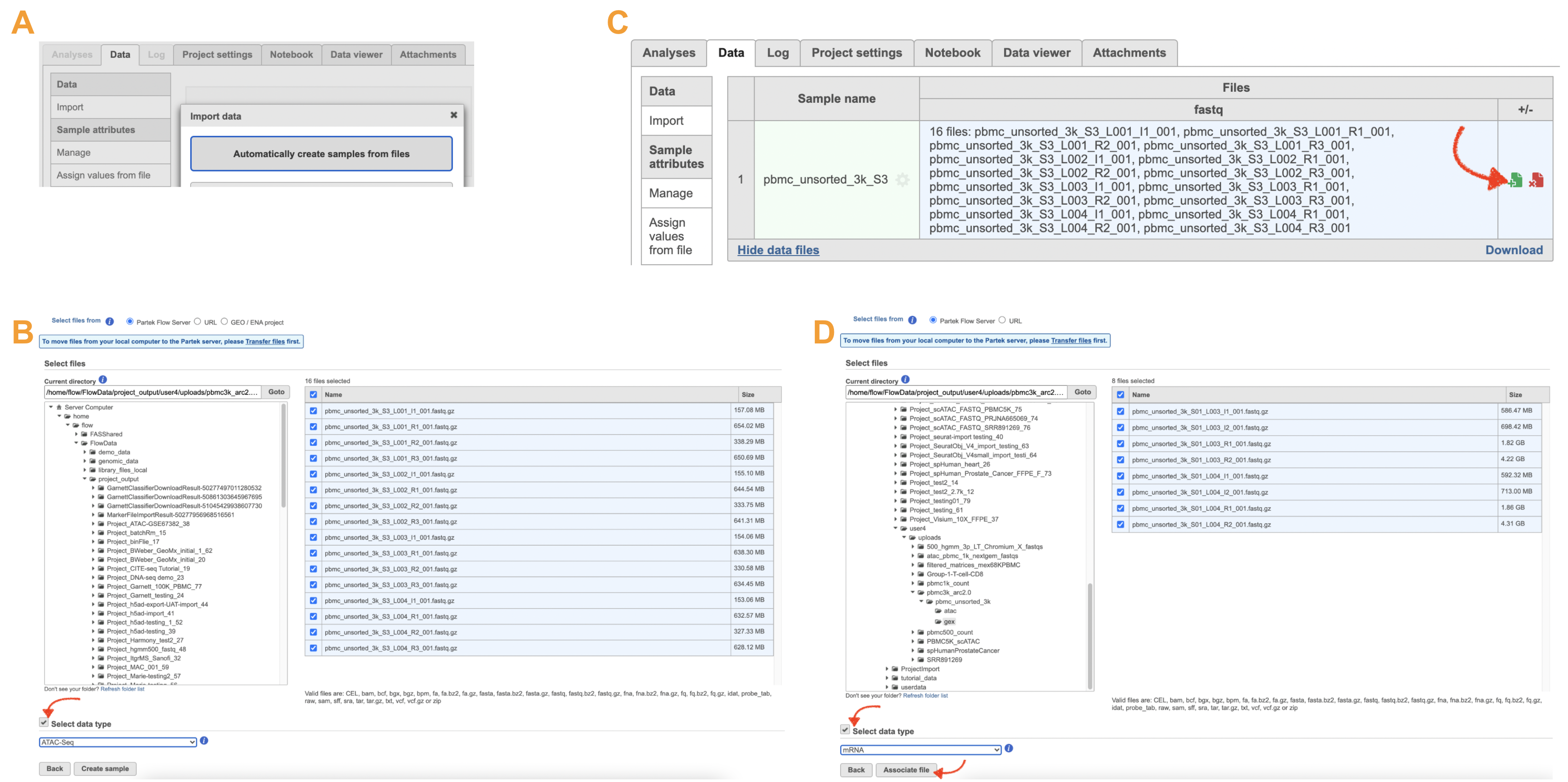
Import ATAC FASTQ files are as straightforward as the (sc)RNA-seq data. However, we have to associate two different types of data together for 10x multiome ATAC + Gene Expression data. It includes two steps: 1). Import ATAC FASTQ files in the new page after clicking on the "Automatically create samples from files" in Data tab (Figure 10A) and select data type from the dropdown list (Figure 10B) to be ATAC-Seq. 2). Move back to the Data tab and display all the files by clicking on the Show data files button at the bottom left of Sample name table. Then go clicking the green + button (Figure 10C)to add RNA FASTQs to the same sample. Similarly, we will select data type from the dropdown (mRNA) before we finish the import process by clicking on the Associate file button (Figure 10D).
| Numbered figure captions | ||||
|---|---|---|---|---|
| ||||
References
https://support.10xgenomics.com/single-cell-gene-expression/software/overview/welcome
https://support.10xgenomics.com/single-cell-atac/software/pipelines/latest/release-notes
- https://support.10xgenomics.com/single-cell-multiome-atac-gex/software/pipelines/latest/what-is-cell-ranger-arc
- https://support.10xgenomics.com/single-cell-multiome-atac-gex/software/pipelines/latest/output/summary
Additional Assistance
If you need additional assistance, please visit our support page to submit a help ticket or find phone numbers for regional support.